The 2025 Millers for Nutrition C-level Meeting held in Mumbai marked a turning point in the global effort to scale up food fortification. Hosted by Strategic Fortification Partner Piramal, the two-day convening brought together industry leaders, policymakers, and partners from seven countries to align on strategy, share progress, and address persistent barriers to fortifying staple foods (1).
This article captures key highlights from the convening, drawing from both high-level discussions and in-depth thematic workshops. It explores the major challenges and innovations shaping testing, premix quality, equipment access, and financing while also outlining the strategic actions that will be taken to support millers and scale impact across markets.
Aligning Across Geographies
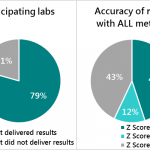
Participants from India, Bangladesh, Ethiopia, Indonesia, Kenya, Nigeria, and Tanzania highlighted common pain points, including limited testing infrastructure, unreliable equipment, and inconsistent premix quality, while also recognizing regional differences in capacity and maturity. These realities informed a shared roadmap for scaling what works and targeting resources where the needs are greatest.
Across countries, testing remains a critical but underdeveloped component of effective fortification. Practices vary widely due to differences in infrastructure, capacity, and policy environments.
In Bangladesh, testing is mostly outsourced, with samples often sent abroad, delaying results and reducing oversight. Seasonal Fortified Rice Kernel (FRK) production limits year-round investment in testing. Local institutions are being considered to strengthen domestic capacity.
India has more advanced infrastructure, but lab inconsistencies persist. This has prompted some producers to invest in internal labs. Platforms like Fortified Rice Traceability (FORTRACE) and a network of reference labs provide a solid foundation for improvement.
In Ethiopia, zinc testing is the focus, while iron is often overlooked. Rapid testing tools are scarce, and standardized protocols are still in development. Local associations are calling for stronger validation and support.
Indonesia stands out with its mature QA systems. Mills conducts regular in-house tests, backed by quarterly third-party verification. Strong relationships with labs and layered quality checks reinforce reliability.
The shared priority across countries is to scale rapid, reliable testing tools supported by clear protocols and training. Digital solutions like iCheck Modular and FORTRACE show promise but will need cross-sector coordination to reach their full potential.
Testing: The Backbone of Fortification

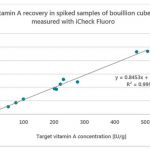
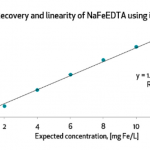
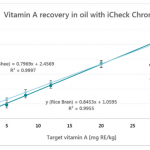
Testing was a dominant theme across sessions. While laboratory infrastructure is expanding, inconsistent protocols and weak enforcement continue to undermine reliability. Stakeholders emphasized finalizing standard procedures for QA, sampling, and data interpretation, as well as validating laboratories across M4N countries. Tools like BioAnalyt’s iCheck Modular are helping lower barriers to adoption with smaller, more affordable reagent kits.
However, Certificate of Analysis (CoAs) are still not universally trusted. Even in markets with accreditation systems, discrepancies often persist due to low test volumes or a lack of specialization. Participants stressed the need for proficiency testing, stronger lab training, and digital dashboards to enhance transparency and trust in results.
Premix Quality: Building Confidence
Millers continue to report concerns around diluted premix, unverifiable CoAs, and inconsistent handling practices. To address these gaps, discussions focused on the value of curated supplier lists, price benchmarks, and third-party quality checks along the supply chain. Trust remains central without reliable inputs; even the best processes fail to deliver fortified products that meet nutritional standards.
Handling at the mill level also matters. Poor storage or blending can compromise even high-quality premix. A call emerged for better training, clearer SOPs, and ultimately, a sector-wide platform to improve traceability and supplier accountability.
Equipment Access and Operational Efficiency
Fortification equipment remains cost-prohibitive for many small and mid-sized millers. Upfront investment, technical complexity, and maintenance needs all contribute to the sense that fortification is burdensome. Proposed solutions included leasing and asset-financing options, bundling equipment sales with training, and setting national technical standards to guide procurement.
The need for hands-on support was clear. From calibration to staff training, millers benefit when equipment providers and technical partners offer ongoing guidance. Operational tweaks such as line balancing or audit support were also flagged as low-cost ways to boost efficiency and confidence in fortification systems.
Financing Fortification: Unlocking Growth
Access to finance remains a major hurdle. While larger companies may have capital, fortification often isn’t a priority without clear incentives. Smaller millers face even tougher challenges accessing credit for premix or equipment. Thematic sessions explored options like performance-based grants, loan guarantees, and de-risking tools to encourage financial institutions to lend.
Importantly, the workshops introduced behavioral insights into the conversation. Understanding how millers assess risk, make sourcing decisions, or adopt new technology is essential. Strategies like peer learning, identity cues, and social proof could increase the likelihood of adoption, especially in voluntary fortification contexts.
A Shared Call to Action

The strength of the coalition lies in its diversity, spanning government, industry, and international NGOs, and the meeting underscored the power of collective action. Participants called on Millers for Nutrition to take the following steps:
- Develop and share reliable SOPs for QA/QC and testing
- Facilitate capacity-building programs on sampling and traceability
- Identify incentives for labs to expand fortification services
- Support innovation in rapid testing tools, particularly for hard-to-measure nutrients
- Enhance access to quality premix and affordable fortification equipment
As the meeting concluded, the energy in the room reflected a shared commitment to move from insight to impact. Fortification is more than a technical solution; it is a strategic opportunity to improve public health through collaboration, innovation, and trust.
The path forward is clear: invest in testing, empower millers, and scale what works. Millers for Nutrition is uniquely positioned to lead this movement and accelerate access to adequately fortified foods across the globe.
To learn more about BioAnalyt’s role in advancing global nutrition, contact us at contact@bioanalyt.com
Reference