The 2025 Millers for Nutrition C-level Meeting held in Mumbai marked a key moment in the global effort to expand access to fortified foods. Hosted by our strategic fortification partner, Piramal Nutrition Solutions, and powered by TechnoServe. The two-day convening brought together senior leaders from seven countries, including millers, policymakers, donors, and technical experts. The goal: align on strategic priorities, celebrate progress, and collaborate to overcome persistent barriers in fortification. With the collective expertise and commitment of partners across the ecosystem, the event offered a platform to explore the major challenges and innovations shaping testing, premix quality, equipment access, and financing while also outlining the strategic actions that will be taken to support millers and scale impact across markets.
Aligning Across Geographies
Delegates from India, Bangladesh, Ethiopia, Indonesia, Kenya, Nigeria, and Tanzania highlighted both shared challenges and regional nuances from limited testing infrastructure to premix quality issues and equipment access. These conversations helped shape a unified roadmap that respects local realities while driving scalable innovation. Testing practices, in particular, emerged as a cross-cutting priority. Infrastructure and enforcement vary widely, yet the need for rapid, reliable tools and consistent protocols remains universal.
In Bangladesh, fortified foods testing is mostly outsourced, with samples often sent abroad, delaying results and reducing oversight. Seasonal Fortified Rice Kernel (FRK) production limits year-round investment in testing. Local institutions are being considered to strengthen domestic capacity.
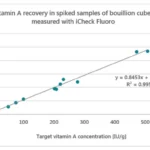
India has more advanced infrastructure, but lab inconsistencies persist. This has prompted some producers to invest in internal labs. A network of reference labs and a pilot digital platforms like Fortified Rice Traceability Application (FoRTrace) and provide a solid foundation for improvement.
In Ethiopia, zinc testing is the focus. Rapid testing tools are scarce, and standardized protocols are still in development. Local associations are calling for stronger validation and support.
Mills in Indonesia conduct regular in-house tests, backed by quarterly third-party verification. Strong relationships with labs and layered quality checks reinforce reliability.
The shared priority across countries is to scale rapid, reliable testing tools supported by clear protocols and training.
Testing: The Backbone of Fortification

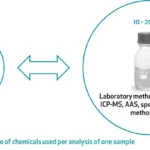
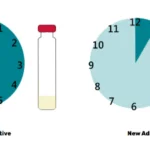
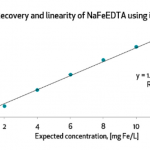
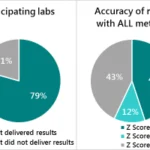
Testing was a dominant theme throughout the convening, reinforcing its central role in fortification efficacy and trust. While infrastructure is steadily expanding, the absence of standardized protocols and consistent enforcement continues to limit effectiveness. Stakeholders emphasized the importance of finalizing guidelines for quality assurance, sampling, and data interpretation. The validation and accreditation of laboratories across Millers for Nutrition countries emerged as a clear next step to bolster confidence in test results. However, Certificate of Analysis (CoAs) are still not universally trusted. Even in markets with accreditation systems, discrepancies often persist due to low test volumes or a lack of specialization. Participants stressed the need for proficiency testing, stronger lab training, and digital dashboards to enhance transparency and trust in results.
Premix Quality: Building Confidence
Consistent access to high-quality premix remains a fundamental concern for many millers. Reports of diluted inputs, unverifiable CoAs, and inconsistencies in handling practices highlight the importance of a more robust and accountable supply chain. Participants emphasized the value of curated supplier lists, clear price benchmarks, and third-party quality validation. At the mill level, storage and blending practices also require attention. Even top-tier premix can be compromised if not handled correctly. This underscores the need for practical training, streamlined SOPs, and digital traceability platforms that promote accountability and quality assurance from end to end.
Equipment Access and Operational Efficiency
Many small and mid-sized millers face steep challenges when it comes to procuring, installing, and maintaining fortification equipment. High upfront costs, complex technical requirements, and limited after-sales support can create a perception that fortification is burdensome. To address this, participants highlighted the need for reliable equipment and process support. Technical partners emphasized the importance of bundling equipment with training and post-sale support. Government standards and process optimization were also recognized as key levers for improved consistency in fortification quality.
Operational efficiency was another focal point. Simple interventions, from calibration assistance to line balancing and technical audits, were highlighted as impactful ways to optimize fortification processes and increase confidence among millers.
Financing Fortification: Unlocking Growth
Access to finance remains a major hurdle. While larger companies may have capital, fortification often isn’t a priority without clear incentives. Smaller millers face even tougher challenges accessing credit for premix or equipment. Thematic sessions explored options like performance-based grants, loan guarantees, and de-risking tools to encourage financial institutions to lend. Challenges such as limited working capital for premix procurement, high upfront costs for equipment, and weak incentives to prioritize fortification were explored. Financing partners and advisors shared models such as performance-based grants and leasing structures to support capital access. A shared takeaway was the need to tie financial incentives to compliance, innovation, and market access milestones.
Importantly, the workshops introduced behavioral insights into the conversation. Understanding how millers assess risk, make sourcing decisions, or adopt new technology is essential. Strategies like peer learning, identity cues, and social proof could increase the likelihood of adoption, especially in voluntary fortification contexts.
A Shared Call to Action
One of the most powerful outcomes of the meeting was the shared sense of momentum and purpose. From government representatives to mill owners, development partners to technical advisors, there was widespread agreement on the path forward. A key outcome of the convening was a shared understanding that isolated efforts are no longer sufficient. What’s needed now is aligned, collective action with clear roles, accountability, and systems that reinforce quality at scale. For the next phase, the focus will be on supporting millers with the practical tools, financing pathways, and behavioral insights needed to make fortification a sustained and scalable reality.
Key actions identified by the coalition include:
-
Developing and disseminating standardized SOPs for QA/QC and testing
-
Facilitating capacity-building initiatives focused on sampling and traceability
-
Incentivizing laboratories to expand fortification services
-
Supporting the innovation and deployment of rapid testing tools, especially for harder-to-measure nutrients
-
Enhancing millers’ access to high-quality premix and affordable fortification equipment
As the convening concluded, participants reaffirmed their collective commitment to move from insight to implementation. Fortification is more than a technical intervention—it represents a strategic opportunity to improve public health, enhance food systems, and empower the private sector to play a leading role in advancing nutrition outcomes. The path forward is clear: invest in testing, empower millers, and scale what works. Millers for Nutrition is uniquely positioned to lead this movement and accelerate access to adequately fortified foods across the globe.
To learn more about BioAnalyt’s role in advancing global nutrition, contact us at contact@bioanalyt.com